Rebuilding Trust in Research: How Data-Driven Metrics Are Shaping a Fairer Future for Clinical Trials
- maninon0
- Oct 21, 2025
- 2 min read
Updated: Oct 22, 2025
What if the next breakthrough therapy worked beautifully, just not for everyone?
That is not a futuristic “what-if.” It is a real risk when studies fail to reflect the people they are meant to help. Across the globe, clinical research still struggles with inclusion, and the trust gap continues to widen, especially in underserved communities.
A 2025 Global Clinical Trials Forum report revealed that only 10% of global trials include meaningful participation from low-income or minority populations, even though these groups shoulder most of the world’s disease burden. It is a stark reminder that science without representation is science with blind spots.
The Trust Deficit Is Still the Hardest Hurdle
Policy updates like the 2025 FDAAA 801 Final Rule and the WHO Global Transparency Initiative have pushed for greater accountability by requiring faster posting of results and clearer consent disclosures. Yet transparency on paper does not always translate to trust in practice.
Nearly 50% of completed studies still do not publish full results within mandated timelines, according to the WHO’s 2025 tracker. That delay breeds skepticism, particularly among communities that already face systemic neglect.
Trust is not rebuilt by regulation alone. It is earned through visibility, communication, and consistent follow-through.
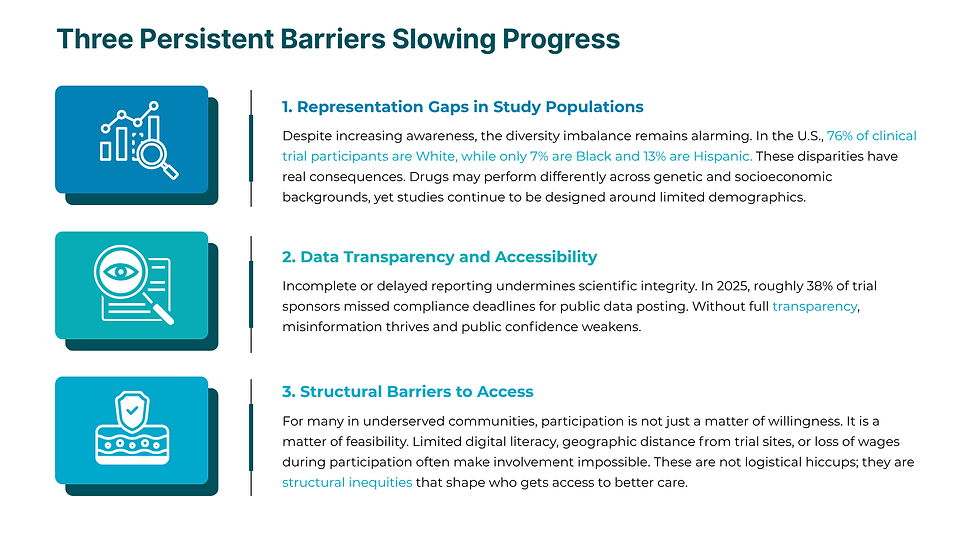
Turning Data Into Trust: How Metrics Drive Clinicoequity
With over 18 million datasets spanning real-world health, trial, and demographic data, Rubix LS is applying a data-driven approach to address these gaps. Using clinicoequity as a guiding framework, the company systematically measures how well research designs serve their intended populations and where they fall short.
Here is how that looks in action:

These steps turn numbers into narratives of inclusion and data into decisions that build trust.

The Road Ahead
The landscape of clinical research is shifting. Regulators are calling for transparency, and communities are demanding fairness. But progress depends on more than compliance. It depends on the courage to measure what truly matters.
Clinicoequity offers a way to do just that: to quantify fairness, track inclusion, and ensure that no population is left out of medical progress.
If you are leading or partnering in research, now is the moment to act.
Visit our site to explore how metric-based strategies can transform the next generation of clinical trials for the communities that need them most.
Together, we can make research not only innovative but just.
References:
U.S. Food and Drug Administration, Center for Drug Evaluation and Research. (2021, February). 2020 Drug Trials Snapshots summary report [PDF]. https://www.fda.gov/media/145718/download
Chodankar, D., & Bhatt, A. (2025). Clinical trial transparency and trust: Bridging the gap between data and disclosure. Perspectives in Clinical Research, 16(3), 109–110. https://doi.org/10.4103/picr.picr_204_25
Studna, A. (2025, September 10). Ensuring digital trial platforms work for underserved communities. Applied Clinical Trials Online. https://www.appliedclinicaltrialsonline.com/view/ensuring-digital-trial-platforms-underserved-communities



