Decentralized Clinical Trials: Benefits, Pitfalls, and Implementation Tips
- maninon0
- Oct 10, 2025
- 4 min read
Clinical trials are instrumental in the development of new treatments and therapies, allowing scientists to develop and test potentially life-saving new drugs and technologies. However, until fairly recently, participating in a clinical trial meant living within a short distance of a hospital or research center where the research was being conducted. This limitation meant some demographics were overlooked or excluded from participation.
Thankfully, new technologies allow high-quality scientific research to be conducted with a fully remote patient pool.
What Are Decentralized Clinical Trials (DCTs)?
Contrary to traditional clinical trials that take place in a singular location, DCTs are conducted in a distributed fashion, allowing patients to participate in their homes, at local healthcare facilities close to them, or even fully remotely via digital platforms.
This level of flexibility creates a fundamental shift in how participants can be recruited and allows people with wildly different abilities and personal circumstances to contribute to medical discoveries. DCTs put patients first, and they often produce high-quality data.
DCTs - A Growing Phenomenon
Pfizer’s 2011 REMOTE clinical trial was a groundbreaking endeavor: it was the first trial to eliminate geography as a factor. Patients were able to enroll, consent, and participate in various study milestones without leaving their homes. Since then, DCTs have exploded in popularity, both out of convenience and necessity.
The COVID-19 pandemic further accelerated the trend, and technologies to support DCTs were crucial to continuing scientific research at a time when social distancing was mandated. During this period, groups like the FDA emphasized the need for flexibility, as well as issuing new recommendations about safety, informed consent, and more, in the new remote-first environment.
The rapid growth and adoption of digital health technologies (DHT) have also played a vital role in the increasing number of DCTs being conducted, with telemedicine platforms, highly accurate wearables, remote monitoring devices, and mobile apps providing researchers with high-quality data without requiring patients to travel to a research center.
Johnson & Johnson’s CHIEF-HF trial, where researchers collected data from step trackers worn by heart failure patients, is often cited as an example of how DHT can collect real-world data in a way that was never possible before.


DCT Common Challenges and Pitfalls
While DCTs have undeniable benefits, there are some additional considerations to acknowledge when planning, including:
Technology Barriers: Participants may not have universal access to the internet or the digital literacy to effectively use some of the more advanced remote tools, creating a new form of exclusion. Elderly patients are an important group to consider here, as well as those in areas with limited broadband access.
Patient Privacy and Security: Trial sponsors should take important steps to protect any health information that is being transmitted through digital platforms or apps. Common cybersecurity risks like data breaches or vulnerable network connections are always a concern, so opting for end-to-end encryption and secure data storage is critical.
Regulatory and Compliance Complexity: When expanding decentralized trials across country borders or larger regions, data integrity and standardization must always be top of mind. This is an added layer of complexity that some research teams fail to account for. Regulations may be wildly different from one location to another, creating further challenges.
Patient Engagement and Retention: Remote trials can eliminate travel burden, but they can also lead patients to feel disconnected from the trial process. Speaking with research coordinators and getting to know the trial staff is often an important part of a patient’s experience. Failing to maintain those lines of communication in a decentralized trial framework can lead to higher drop-out rates.
DCTs - How CROs Can Help
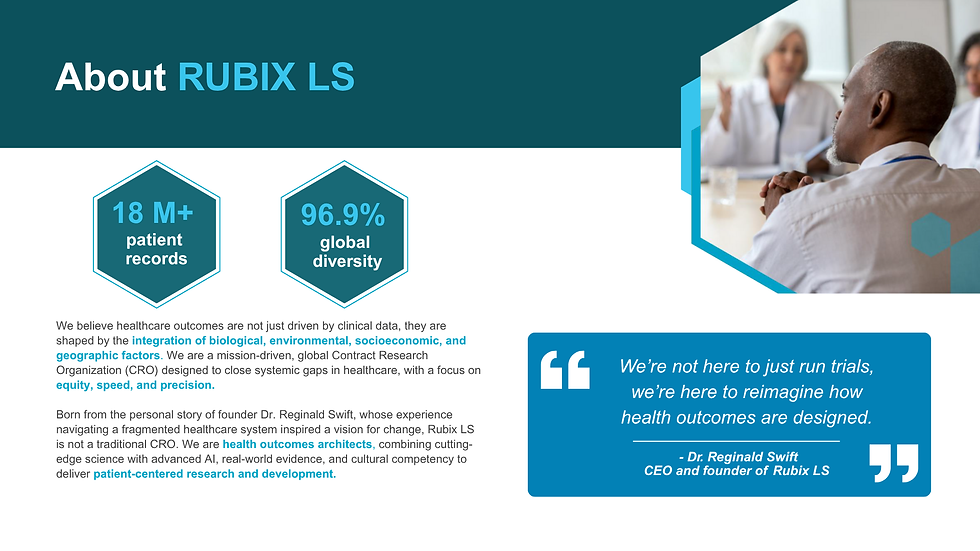
At Rubix LS, we specialize in designing, managing, and executing decentralized clinical trials. Our innovative approach integrates environmental, geographic, socioeconomic, and biological factors to ensure that each trial is built on a foundation of diversity and puts patient needs first.
We utilize a vast database of over 18 million patient records, providing proven expertise in both recruiting and retaining historically overlooked populations. Utilizing a proprietary AI-based approach, the Rubix LS platform allows researchers to recruit more efficiently, improve the predictability of outcomes, and discover biomarkers with greater accuracy. We fully support flexible, decentralized trials that leverage the latest digital health technologies and mobile clinical units, expanding the reach of trials to underserved communities.
Our extensive experience with the many global regulatory bodies, such as the FDA, EMA, PMDA, CFDA, and ANVISA, means we are positioned to ensure compliance across a wide range of jurisdictions. Our Patient X platform, which facilitates seamless collaboration across patients, providers, and sponsors, enhances engagement and boosts retention, giving each trial the best possible chance at success.


The Future Is Decentralized
At Rubix LS, we design and implement decentralized trials that work for both sponsors and trial participants. We take a comprehensive approach that leverages cutting-edge tech, a deep understanding of the regulatory landscape, and a sensitive approach to patient needs that ensures a research experience that is secure, accessible to all, and scientifically rigorous.



